Immunoglobulin E (IgE) Defined
About IgE
In this post, we will define Immunoglobulin E (IgE). IgE produced by the immune system is an important antibody for your body’s immune defenses.
Immunoglobulin E (IgE) Defined
If you have an allergy, your immune system overreacts to an allergen by producing antibodies called Immunoglobulin E (IgE). These antibodies significantly travel to cells that release chemicals, causing an allergic reaction. This reaction usually causes symptoms in the nose, lungs, throat, or on the skin.
Each type of IgE has specific chemical “radar” for each type of allergen. The immune system has many types of IgE. Thus this is why some people are only allergic to cat dander (they only have the IgE antibodies specific to cat dander. Others people have allergic reactions to multiple allergens because they have many more types of IgE antibodies.
Immunoglobulin E is one of the five classes of immunoglobulins (IgM, IgG, IgD, IgA, IgE). IgE has a unique chemical structure and an array of physiological functions, such as Type I hypersensitivity reactions, parasitic infections, autoimmune processes, and venom protection.
At the Molecular Level
The Fc Region and Receptors
All immunoglobulin molecules have a common structural framework characterized by two identical small polypeptide chains (light chains) and two identical large polypeptide chains (heavy chains). The fragment crystallizable region called the ( Fc region). Specifically, the tail region of an antibody interacts with cell surface receptors called Fc receptors. The chemical reaction allows antibodies to activate the immune system and nuetrilize the antigen. The Fc region of the antibody has two heavy protein chains. These protein chains are for the purpose of binding to cellular targets. Afterwards, neutralizing antibody ( NAb) preventing the particle from interacting with its host cells it might infect and destroy.
The Fab Region
The fragment antigen-binding region (Fab region) is a region on an antibody that binds to antigens. Proteins composed of one constant and one variable domain of each of the heavy and the light protein chain. The Fab region of the antibody recognizes the antigen. It contains two heavy chains continuous with the Fc region and two light chains.
How the Fc and Fab regions work together
At the antigen binding sites, both polypeptide chains contain variable regions whose amino acid sequences are altered for a given antigen. The variable heavy and light chains are organized into layers. Thus, these layers of antiparallel B-pleated protein sheets allow the antigen to bind between these three-dimensional immunoglobulin folds. The rest of the polypeptide regions are known as constant regions, and they are held together by strong dipeptide bonds to stabilize the two chains.
Typical IgE molecule Protein
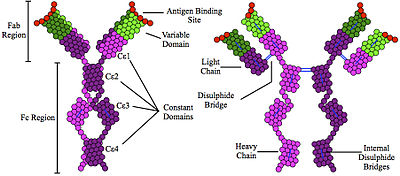
Protein diagram Immunoglobulin E (IgE) Defined
More articles to read
How Immunoglobulin E (IgE) Functions in Your Body – The Antibody Involved in the Allergic Response