About RSV Respiratory Syncytial Virus
Respiratory syncytial virus
Respiratory syncytial virus, or RSV, is a common respiratory virus that usually causes mild, cold-like symptoms. Most people recover in a week or two, but RSV can be serious. Infants and older adults are more likely to develop severe RSV and need hospitalization. If you are age 60 or older, a vaccine is available to protect you from severe RSV.
Last fall, when RSV and flu came roaring back from a prolonged and erratic hiatus. COVID was still killing thousands of people world wide each week. Many of the leading infectious-disease experts offered the world a glimmer of hope. The hospitals worldwide would be overwhelm, they predicted, was probably temporary—viruses making up ground they’d lost during the worst of the pandemic. Next year would be better.
And so far, this year has been better. Some of the most prominent and best-tracked viruses, at least, are behaving less aberrantly than they did the previous autumn. Although neither RSV nor flu is particularly mild this year.
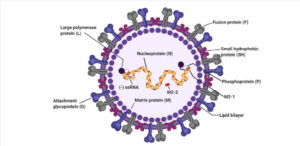
About RSV Respiratory Syncytial Virus – Model of RSV virus
Who Is at Risk for Respiratory Syncytial Virus
RSV immunizations are recommended for these groups:
infants and young children
- Two RSV vaccines (Arexvy by GSK and Abrysvo by Pfizer) have been licensed by FDA and recommended by CDC for adults ages 60 and older, using shared clinical decision-making.
- One RSV vaccine (Abrysvo by Pfizer) has been licensed and recommended during weeks 32 through 36 of pregnancy to protect infants.
- An RSV preventive antibody has been licensed and recommended for infants and some young children.
-
RSV can be dangerous for infants and young children. Each year in the United States, an estimated 58,000-80,000 children younger than 5 years are hospitalized due to RSV infection. Children at greatest risk for severe illness from RSV include the following:
- Premature infants
- Infants up to 12 months, especially those 6 months and younger
- Children younger than 2 years with chronic lung disease or congenital (present from birth) heart disease
- Children with weakened immune systems
- Children who have neuromuscular disorders, including those who have difficulty swallowing or clearing mucus secretions

Be aware of the risk factors of Respiratory Syncytial Virus
Older Adults and Adults with Chronic Medical Conditions
RSV infections can be dangerous for certain adults. Each year, between 60,000-160,000 older adults in the United States are hospitalized with RSV. Consequently, 6,000-10,000 adults die due to RSV infection. Adults at highest risk for severe RSV infection include:
- Older adults
- Adults with chronic heart or lung disease
- Adults with weakened immune systems
- Adults with certain other underlying medical conditions
- Adults living in nursing homes or long-term care facilities
The Immunology of RSV Vaccine History
About how the RSV Respiratory Syncytial Virus vaccine, was developed. Attempts to develop an RSV vaccine began in the 1960s with an unsuccessful inactivated vaccine developed by exposing the RSV virus to formalin (formalin-inactivated RSV (FI-RSV)). Specifically, two children died of lethal lung inflammation after vaccination. Thus this disaster slowed vaccine development for many years.
By 1998
A 1998 paper reported that research had advanced greatly over the previous 10 years. A 2019 paper similarly claimed that research toward developing a vaccine had advanced greatly over the prior 10 years, with more than 30 candidates in some stage of development. The same study predicted that a vaccine would be available within ten years. Candidates included particle-based vaccines, attenuated vaccines, mRNA vaccines, protein subunit vaccines, and vector-based vaccines.
By 2013
A 2013 study detailed the crystal structure of the RSV fusion (F) protein. Particularly, this study found the F protein stability improvement. This provided the basis for finding the most effective F protein constructs, which are used in RSV vaccines. Subsequently to develop its vaccine, Pfizer engineered 400 different F protein constructs to identify the most immunogenic protein. In the same fashion, they constructed a bivalent RSV prefusion F protein vaccine.
Summary of results 2023
In February 2023, Pfizer published the results of the phase III study. This Study had close to 25,000 participants age 60+. One dose of the Arexvy vaccine provided 94% efficacy against severe RSV pneumonia and 72% efficacy against RSV acute respiratory infection. An advisory panel to the FDA recommended approval of the vaccine in February 2023.
In April 2023, the Committee for Medicinal Products for Human Use of the European Medicines Agency (EMA) recommended to grant a marketing authorization for Arexvy for the prevention of RSV lower respiratory tract disease in adults 60 years of age or older after review under EMA’s accelerated assessment program.
References
Fick M (9 March 2023). “GSK expects US launch of RSV vaccine with no supply issues”. Reuters. Archived from the original on 12 April 2023. Retrieved 12 April 2023.
Bendix A (28 February 2023). “Paving the way for the world’s first RSV vaccine, FDA advisers recommend shot from Pfizer”. NBC News. Archived from the original on 28 February 2023. Retrieved 28 February 2023.
“Arexvy: Pending EC decision”. European Medicines Agency. 26 April 2023. Archived from the original on 27 April 2023. Retrieved 27 April 2023. This text copied from copyrighted European Medicines Agency. Reproduction authorized provided the source is acknowledged.
“First vaccine to protect older adults from respiratory syncytial virus (RSV) infection”. European Medicines Agency (EMA) (Press release). 26 April 2023. Archived from the original on 27 April 2023. Retrieved 27 April 2023.
Clinical trial numbers
NCT04886596 for “Efficacy Study of GSK’s Investigational Respiratory Syncytial Virus (RSV) Vaccine in Adults Aged 60 Years and Above” at ClinicalTrials.gov
NCT04732871 for “Immunogenicity, Safety, Reactogenicity and Persistence of an Investigational Respiratory Syncytial Virus (RSV) Vaccine in Adults Aged 60 Years and Above” at ClinicalTrials.gov
NCT04841577 for “A Study on the Immune Response and Safety Elicited by a Vaccine Against Respiratory Syncytial Virus (RSV) When Given Alone and Together With a Vaccine Against Influenza in Adults Aged 60 Years and Above” at ClinicalTrials.gov
NCT05035212 for “Study to Evaluate the Efficacy, Immunogenicity, and Safety of RSVpreF in Adults. (RENOIR)” at ClinicalTrials.gov
NCT04424316 for “A Trial to Evaluate the Efficacy and Safety of RSVpreF in Infants Born to Women Vaccinated During Pregnancy” at ClinicalTrials.gov